Quick Specs
Inform critical decisions with Ansys Granta Materials Data. Leverage a library of materials data with your relevant Granta software.
Our range of Materials Data are created and curated by our world-class team of materials specialists to be used with our Granta MI, Selector and EduPack tools.
Choose the right Materials Data for your needs
All Granta software includes a set of Core Materials Data and their properties. This can be supplemented with a wide spectrum of Advanced Materials Data for different industries and materials types. Access design, supplier, or test data or standards and specifications – helping to inform critical decisions across design, materials selection and more.

Inform critical decisions with Ansys Granta Materials Data. Leverage a library of materials data with your relevant Granta software.
Searching for a replacement to PA66 to solve a supply issue.

By using our Core and Advanced Materials Data with Ansys Granta Selector, alternative polymers can be found to solve severe supplier shortages.
When global supply issues resulted in PA66 being difficult to source, our customers turned to Ansys Granta to seek a solution.
By accessing Granta’s unique MaterialUniverseTM polymers data, which is part of our Core Generic data offering with each of our products, materials experts were able to access critical PA66 data.
Using Granta Selector they were able to quickly find a range of viable alternative polymers given the price, flexural modulus, flammability constraints and for its suitability for injection molding.
Finally, using our Advanced Polymers Materials Data, the individual grades from suppliers were found. Resulting in an actionable alternative polymer found, at a lower cost.
July 2023
We're taking the latest data from leading material producers, including detailed high-temperature alloys - ideal for power generation, aerospace, and gas turbine applications.
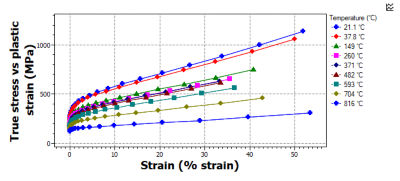
Our new High-Temperature Alloys Data table focuses on high-temperature mechanical and thermal properties for engineering alloys. This includes GE-licensed materials data for 75+ alloys with temperature-dependent data on thermal, tensile, creep, fatigue, and fracture performance.

Now includes new Ansys test data and data directly from key polymer producers. We've introduced unique attributes to improve searchability for recycled and biodegradable polymers. Simulation readiness is enhanced with new data on mechanical and thermal performance vs. temperature, electromagnetic and optical behavior.

Material environmental impact values in MaterialUniverse™ is updated for CO2 footprint and embodied energy. JAHM Curve Data includes 2,500+ new materials plus improved stress-strain, creep, and strength data coverage. A new optical subset is introduced for materials with non-linear refractive index data.
With a broad range of materials information, Granta Materials Data provides data that engineers need – when and where you need it. Our unrivalled material library serves as a complete and cost-effective solution. Users can leverage the latest materials data to search, compare and analyze. By using our Granta software products, this materials data can be seamless export or integrated into a wide range of CAD, CAE and PLM solutions.
Featuring a comprehensive library of materials data
Core Data:
Advanced Data:
Access Granta's unique generic database of engineering, economic and eco property profiles for thousands of materials. MaterialUniverseTM allows for a like-to-like comparison across material and processing possibilities.
Specialist Temperature-dependent property data for thousands of datasheets. Ideal as input data for simulation. Included as part of our Granta Core Data.
Our Advanced Metals Data includes a combination of Design, Test and Standards and Specifications data. Global Metals Specifications incorporates materials data from ASM International, the German Steel Institute, UK Steel and WMI.
Design data for Boiler and pressure vessel data from ASME.
Creep and fatigue data from Japan’s NIMs – exclusively for Granta MI Enterprise.
Standards and specifications data on powder metallurgy and test data for sheet steels is also available.
This set of supplier grade data from well-respected spec sheets, gives you access to hundreds of thousands of resins and tens of thousands of polymer additives. This data enables designers to compare the properties and processing of plastics and elastomers, in addition to selecting the appropriate additives.
With our composite data, users gain access to data from manufacturers and leading research projects including Mil-Handbook-17 and Firehole Composites for standards and specifications. Traceable composite data from the NCAMP and AGATE projects to support qualification, equivalency, and design with the Composite QED, is only available for our Granta MI Enterprise product.
Leverage comprehensive supplier grade data on additive manufacturing machines and compatible materials from Senvol DatabaseTM.
With our aero data, users have access to authoritative design data from MMPDS, in addition to specialist data on relevant coatings.
Analyze information on restricted substance risk of materials and processes, environmental impact and supply risk. Available with the Restricted Substance solution with Granta MI Enterprise.
Specialist data with ASM Medical Materials for cardiovascular, orthopaedic, neurological, surgical, ENT, urological devices available as part of the Medical Bundle with Granta MI. Granta Selector has access to the on-line version.
Human Biological Materials including mechanical properties of human tissues, including bone, cartilage, ligaments, tendons, circulatory and dental tissues is available for Granta MI Enterprise only.
European design-strength design data on aerospace metallic materials. Purchasable as a standalone option within Granta MI or Granta Selector.
Specialist data for key materials indicators and background information from ecoinvent. Purchasable as a standalone option within Granta MI or Granta Selector.
The Electromagnetics data set includes information on 7,200+ material records for low and high frequency electromagnetic applications – printed circuit board materials, soft magnetic alloys, permanent magnets and EM shielding/absorbing materials.